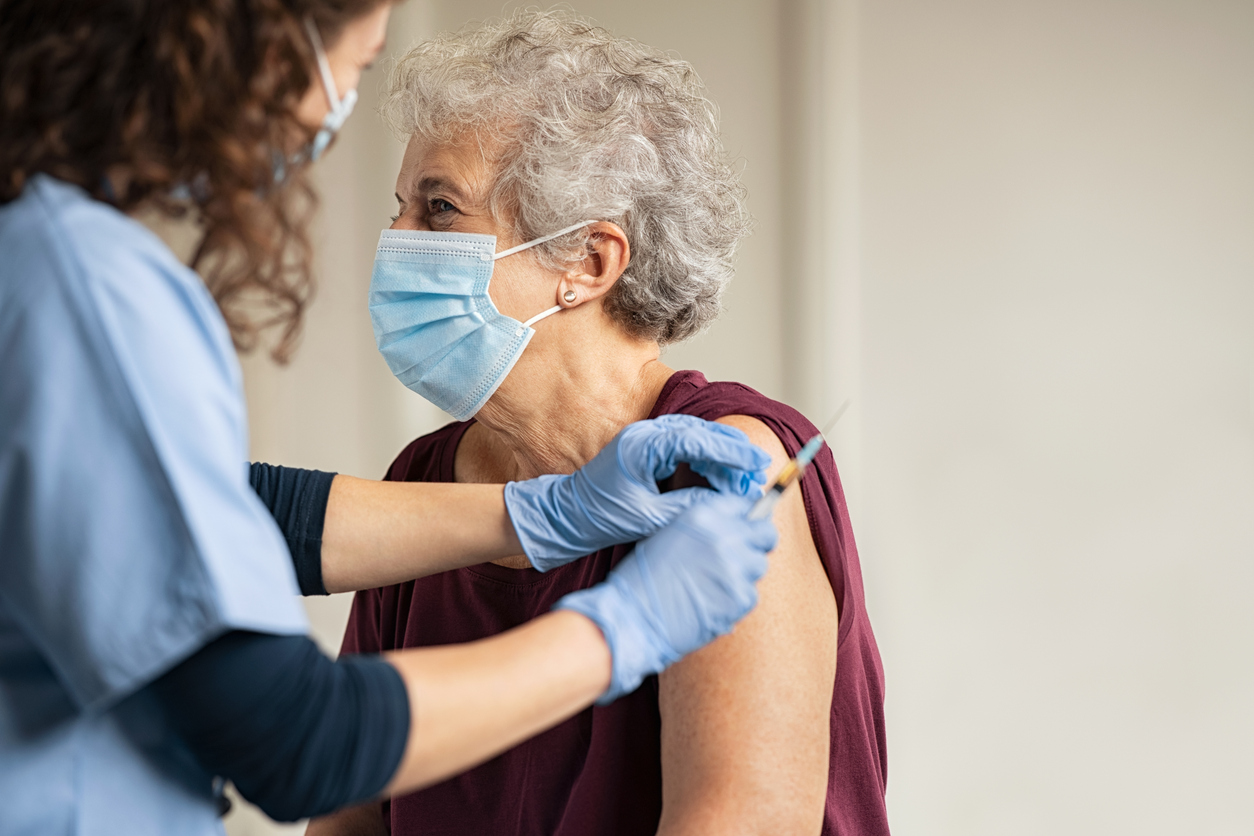
WHO Calls for Actions to Encourage Acceptance and Uptake of COVID-19 Vaccines
December 16, 2020| |
As vaccines for COVID-19 reach high efficacy rates and with Britain's approval of Pfizer-BioNTech vaccine for emergency use, the World Health Organization (WHO) pinpoints the next challenge: public acceptance of the vaccine.
After the vaccine approval, a system for mass production for sufficient supply, efficient rollout, and equitable access would be laid out. Strategies to drive acceptance and uptake of the vaccines are vital in the process. Thus, the WHO Technical Advisory Group on Behavioral Insights and Sciences for Health released a report that details the factors that drive people's behavior when it comes to vaccines: an enabling environment, social influences, and motivation. When these factors are properly addressed, communities would be encouraged to accept and take up vaccination.
The recommendations of the report include the following:
- The vaccination must be easy, quick, and affordable.
- Vaccines must be available in safe, familiar, and convenient locations such as "drop-in" clinics where people usually go.
- Rollout plans should be combined with a targeted, credible, and clear information drive on the importance and benefits of vaccination explained by trusted sources.
WHO stressed that communicating consistently, transparently, empathetically, and proactively about uncertainty, risks, and vaccine availability is necessary to build public trust.
Get more information from WHO.
| |
You might also like:
- COVID-19 Resource
- National Survey Says Most Americans Willing to Vaccinate Against COVID-19
- Human Trials Begin for COVID-19 Plant-Based Technology Vaccine
Biotech Updates is a weekly newsletter of ISAAA, a not-for-profit organization. It is distributed for free to over 22,000 subscribers worldwide to inform them about the key developments in biosciences, especially in biotechnology. Your support will help us in our mission to feed the world with knowledge. You can help by donating as little as $10.
-
See more articles:
-
News from Around the World
- ISAAA Short Course Highlights Relevance of Integrating Research, Science-based Regulation, and SciCom in Sustainable Agri Dev't
- ISAAA Shares 2019 Global Biotech Crop Adoption to Stakeholders in the Philippines and Arab Region
- Give a Gift of Knowledge
- Webinar: Biotech Crop Adoption in 2019 and Latin America's Experience
- NBMA Validates National Biosafety Guidelines on Gene Editing in Nigeria
- University of Florida Study Uncovers Vanilla DNA Mystery
- RNAi Pesticides Could Be the Next Big Tool in Agriculture
- US FDA Has Approved First GM Pig for People with Red Meat Allergies
- Chinese Scientists Develop Rice Variety Using Ion Beam Technology
- GM Tomato Enriched with L-DOPA Offers Affordable Medicine for Parkinson's Disease
-
Research Highlights
- Soybean Gene Overexpression Offers Resistance to SDS
-
Plant
- Biotech Research Perceived With Caution Globally, but Most Support Gene Editing for Therapeutics
-
Health
- WHO Calls for Actions to Encourage Acceptance and Uptake of COVID-19 Vaccines
-
Read the latest: - Biotech Updates (December 17, 2025)
- Gene Editing Supplement (December 17, 2025)
- Gene Drive Supplement (February 22, 2023)
-
Subscribe to BU: - Share
- Tweet