India Eases Process for Release of Genome Edited Plants Without Foreign DNA
May 25, 2022| |
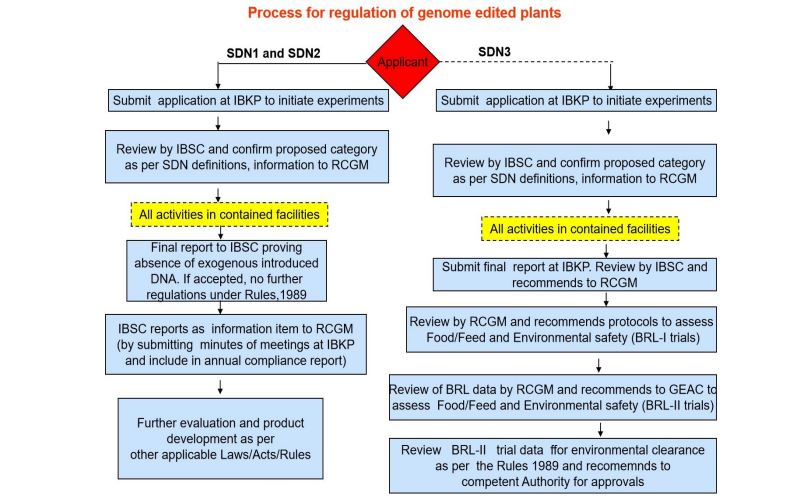
After extensive deliberations of genetic engineering experts in India, the government released the final guidelines for the safety assessment of genome-edited plants on May 17, 2022. According to the office memorandum released by the Department of Biotechnology, the guidelines serve as a road map for the development and sustainable application of genome editing, including the regulatory pathways to be taken for the release of genome-edited plants.
The guidelines state that genome-edited plants that do not contain foreign DNA are exempted from Rules 1989, which are implemented for genetically engineered plants by the Genetic Engineering Appraisal Committee (GEAC). The Institutional Biosafety Committee will monitor the genome-edited plants under containment until they are free from foreign DNA. The new guidelines are based on the recommendations of the Department of Biotechnology, Ministry of Science and Technology and the Department of Agriculture Research and Education, Ministry of Agriculture and Farmers Welfare.
Read the guidelines posted on the website of the Department of Biotechnology.
| |
You might also like:
- India Exempts Genome-Edited Plants from Biosafety Assessment
- Biotech Country Facts and Trends: India
- Genome Editing for Sustainable Agriculture
Biotech Updates is a weekly newsletter of ISAAA, a not-for-profit organization. It is distributed for free to over 22,000 subscribers worldwide to inform them about the key developments in biosciences, especially in biotechnology. Your support will help us in our mission to feed the world with knowledge. You can help by donating as little as $10.
-
See more articles:
-
News from Around the World
- FAO DG Qu Dongyu: It's Time to Transform Agrifood Systems
- Oats Genome Explains Why the Popular Cereal Could be Suitable for People with Celiac Disease and Gluten Intolerance
- DA PhilRice Ready to Deploy Golden Rice Seeds to Farmer Cooperative
- European Commission Approves Two GM Crops for Food and Feed
-
Research Highlights
- Location of SunUp's Transgenic Insertions Affirms It's Still Safe for Consumption
-
Plant
- India Eases Process for Release of Genome Edited Plants Without Foreign DNA
- Philippines Releases Regulations for Gene-edited Plants
- Health Canada Issues Gene Editing Guidelines, Encourages Transparency
-
Read the latest: - Biotech Updates (December 10, 2025)
- Gene Editing Supplement (December 17, 2025)
- Gene Drive Supplement (February 22, 2023)
-
Subscribe to BU: - Share
- Tweet