COVID-19 Test Uses CRISPR and Smartphone Camera
December 9, 2020| |
A team of scientists at Gladstone Institutes, University of California, Berkeley (UC Berkeley), and the University of California, San Francisco (UCSF) has developed the technology for a CRISPR-based test for COVID-19 that uses a smartphone camera to provide accurate results in under 30 minutes. The Gladstone team led by Dr. Melanie Ott and Parinaz Fozouni worked with UC Berkeley bioengineer Dr. Daniel Fletcher and Dr. Jennifer Doudna, senior investigator at Gladstone, professor at UC Berkeley, president of the Innovative Genomics Institute, and investigator of the Howard Hughes Medical Institute.
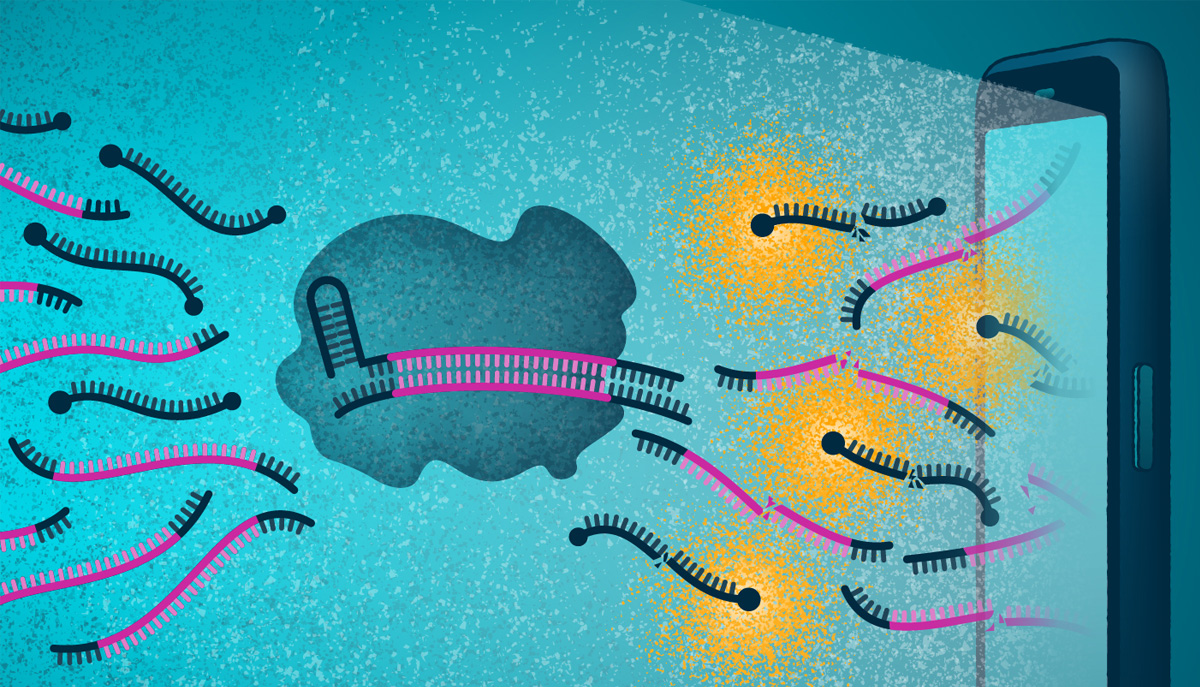
Current COVID-19 tests quantitative PCR which is the gold standard of testing. However, this technique requires DNA. Coronavirus is an RNA virus, which means that to use the PCR approach, the viral RNA must first be converted to DNA. All CRISPR diagnostics to date require the conversion of viral RNA to DNA which is time-consuming and complex. In contrast, this novel approach skips all the conversion and amplification steps, using CRISPR to directly detect the viral RNA.
Instead of the well-known protein Cas9 which cleaves DNA, this new test uses the Cas13 protein which cleaves RNA. The Cas13 protein is combined with a reporter molecule that glows when cut and then mixed with a patient's nasal swab. The sample is placed in a device attached to a smartphone. If the sample contains RNA from SARS-CoV-2, Cas13 will be activated and will cut the reporter molecule, causing the emission of a fluorescent signal. Then, the smartphone camera, essentially converted into a microscope, can detect the fluorescence and report that a swab tested positive for the virus.
The scientists tested their device using patient samples and confirmed that it could provide a very fast turnaround time of results for samples with clinically relevant viral loads. In fact, the device accurately detected a set of positive samples in under 5 minutes. For samples with a low viral load, the device required up to 30 minutes to distinguish it from a negative test.
For more details, read the article on the Gladstone Institutes website.
| |
You might also like:
- Pocket K No. 58: COVID-19 Treatment Efforts Using Plant Technologies
- Digital CRISPR-Cas-Assisted Assay for Rapid SARS-CoV-2 Detection
- UConn Researcher Develops Simple, Low-Cost CRISPR-based Diagnostic Test for COVID-19
Biotech Updates is a weekly newsletter of ISAAA, a not-for-profit organization. It is distributed for free to over 22,000 subscribers worldwide to inform them about the key developments in biosciences, especially in biotechnology. Your support will help us in our mission to feed the world with knowledge. You can help by donating as little as $10.
-
See more articles:
-
News from Around the World
- International Study Decodes the Genome of 15 Wheat Varieties
- International Animal Biotech Webinars Wrap Up for 2020
- ISAAA Webinar: Global Status of Biotech Crops and the Philippine Adoption Experience
- Locust Genome Could Help Find Solutions to Pest's Swarming Behavior
- International Research Team Publishes Barley Pan-Genome
- Filipino Researchers Develop Motion Tracker for Eggplant's Most Vicious Pest
- Biotech Maize Area in Vietnam Expands to 92,000 Hectares
- Research Team Discovers Strategy to Make Plants More Salt Tolerant
-
Research Highlights
- Ectopic Expression of a Grape Nitrate Transporter Boosts Nitrogen Use Efficiency in Arabidopsis
- GM Birch Tree Study Shows Promising Results Against Insect Herbivores
-
Plant
- Two Studies Reveal Retron's Function and its Genome-editing Potential
-
Health
- COVID-19 Test Uses CRISPR and Smartphone Camera
-
Read the latest: - Biotech Updates (January 28, 2026)
- Gene Editing Supplement (January 28, 2026)
- Gene Drive Supplement (February 22, 2023)
-
Subscribe to BU: - Share
- Tweet