Biotech Offers Promise in Global Livestock Industry
| |
Switching off a gene in animals using a technology called CRISPR can make them produce more muscles, leading to meatier animal food products. This is according to Dr. Alison Van Eenennaam, animal biotechnology and genomics professor at the University of California, Davis, USA, during her talk about the global overview of animal biotechnology applications in the livestock industry.
The International Service for the Acquisition of Agri-biotech Applications, Inc. (ISAAA Inc.), hosted the webinar on January 31, 2023, in partnership with Winrock International through the Building Safe Agricultural Food Enterprises (B-SAFE) project. Over 200 individuals from the Philippines and abroad joined the webinar via Zoom and Youtube.
“Genetic improvement of both plants and animals has been an important driver of agricultural sustainability,” said Prof. Van Eenennaam. However, the rate of commercialization and adoption of genetically engineered (GE) animals is way slower than the uptake of GE crops. To date, only two GE food animals are available commercially in the US—the fast-growing AquAdvantage salmon and the hypoallergenic Galsafe pig. The slow progress in the release of GE animals is attributed to the long road to market involving rigorous research and regulatory hurdles.
Animal improvement has been easier in recent years with new breeding innovations. CRISPR and other techniques collectively known as genome editing create a cut to a specific spot in the DNA without necessarily inserting a gene from another organism. Through these tools, genes can be deactivated to express desirable traits, such as disease resistance, heat tolerance, and improved yield.
Meatier chow
One of the most promising breakthroughs of genome editing in animals is the improved muscle yield in common animal meat sources such as cows, pigs, and fish. Scientists copied a naturally occurring mutation in animals that can lead to more muscles. They achieved this by disabling a protein produced in muscles called myostatin. This small change in the genome can lead to up to 30% more muscle yield in livestock animals.
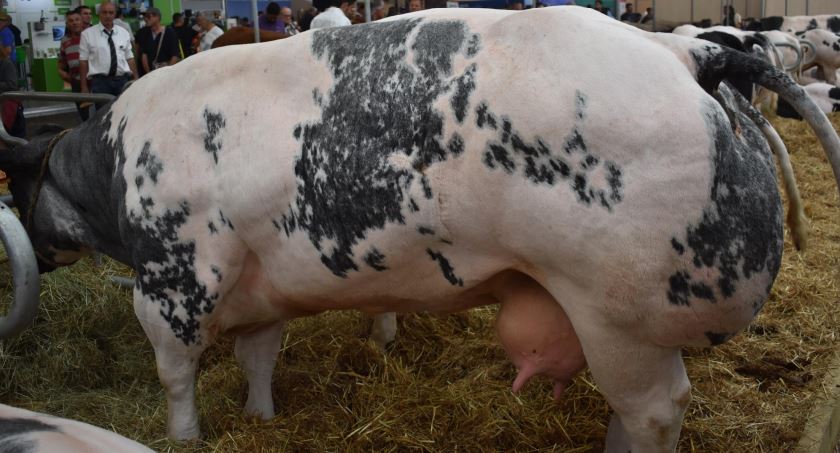
In Japan, experts have doubled the muscle yield of red sea ream and puffer fish. These genome-edited fish are now available in the Japanese markets after they have been recognized as no different from conventionally-bred fish. Researchers in Brazil and Argentina also used the same technique in Tilapia, but it has yet to reach commercialization.
Healthier pigs
Genome editing has provided a potential solution to porcine reproductive and respiratory syndrome (PRRS), a widespread viral disease affecting pigs in many regions worldwide. By simply inactivating a gene, the pigs become resistant to the virus. “As a geneticist, making animals resistant to diseases is a better option than treating animals with antibiotics or chemicals,” Dr. Van Eenennaam emphasized.

Younger meat
Male pigs are prone to becoming aggressive as they age due to the excessive production of sex hormones. Too much testosterone also leads to boar taint, characterized by an unpleasant taste in pork.
Through genome editing, the pigs have been bred to stay effectively adolescent, bypassing the tendency for aggression and boar taint production.
Shinier coat
Animal improvements to withstand climate change conditions are also in the pipeline. Tropically adapted cattle breeds exhibit slick hair caused by a natural mutation in the prolactin receptor gene.
Researchers mimicked this mutation using CRISPR technology to develop cattle that can better adapt to warmer climates. This technique led to animal body temperatures that are 1 degree lower than the conventionally bred cattle.
Safer dairy cattle
Genome editing also addresses animal welfare concerns. Dehorning is a common practice in dairy cattle to prevent injuries to the herds and farm workers. This is done through invasive methods, such as the use of hot iron, chemicals, or saw. This concern led Acceligen to produce a genome-edited bull for breeding hornless offspring. The growth, health, milk, and meat of the offspring were observed from birth to death and no difference was found between the hornless ones and the horned controls.

With the promising benefits of genome editing, one question remains — will genome-edited animals also tread the long road to market, just like the first generation of biotech animals?
“The fate of animal biotech will depend on a harmonized, risk-based regulatory framework that enables international trade of products (milk, meat, eggs), and genetic materials (gametes and embryos),” Dr. Van Eenennaam explained.
Philippine scenario
In the Philippines, researchers at the Philippine Carabao Center are using somatic cell nuclear transfer technology to complement other existing reproductive tools for buffaloes. They have successfully produced buffalo clone embryos, according to Dr. Marvin A. Villanueva, who leads the Lisvestock Biotech Center in the country and is one of the speakers in the webinar.
The ISAAA Inc.-B-SAFE webinar provided insights on biotechnology in support of the government’s efforts to formulate regulations for genetically engineered and gene-edited animal products. B-SAFE Project’s Chief of Party Dr. Ramon Clarete called for a collaborative effort to help bring animal biotech regulations forward in the House of Representatives. When the regulations are in place, these will facilitate the access of Filipino scientists to cutting-edge technologies and products from other countries and help the Philippine livestock research and industry address the current challenges.
This article was first published in Agriculture Magazine. Watch the webinar on demand at the ISAAA Inc. website.
| Newer Post | Archive | Older Post |
Science Speaks is ISAAA Inc.'s official blog. Weekly blog articles, authored by ISAAA writers, partners, and invited contributors, aim to help share, disseminate, and promote scientific knowledge and its vital role in achieving global agricultural sustainability and development. Your support to Science Speaks will help us achieve this goal. You can help us by donating as little as $10.

