ISAAA Policy Brief Tackles Importance of Science-based and Case-by-case Risk Assessment for Gene Drives
| |

ISAAA Inc., together with the Outreach Network for Gene Drive Research launched a policy brief that tackles the importance of science-based and case-by-case risk assessment for gene drives. "Risk Assessment for Gene Drive Organisms" is the first in a series of policy briefs that aim to present the proposed policy options that address issues relating to gene drive technology.
Gene drive is a genetic phenomenon that occurs in nature and causes a selected trait to spread rapidly through a species by way of sexual reproduction over generations, potentially becoming increasingly common within a specific species. Scientists are developing gene drive systems to replicate this natural phenomenon to help tackle public health concerns from vector-borne diseases such as malaria and dengue fever. Through this process, a desired change is passed on to up to 100% of offspring, rather than the usual rate of 50%. This technology is currently under research, and the risks and benefits of each potential application are being thoroughly investigated.
The policy brief answered the following:
- What is a risk assessment?
- Who oversees gene drive research?
- Are we ready to assess the risk and benefits from gene drive technologies?
Risk assessment identifies potential pathways to harm that could lead to adverse health or environmental impacts. It evaluates the likelihood and magnitude of such potential harm occurring and highlights any further elements of uncertainty. The policy brief points out that risk assessment can be carried out at different points in the development process of a gene drive organism. However, every risk assessment has different steps and the risk associated with any gene drive research, in particular for field evaluation, must be assessed on a case-by-case basis.
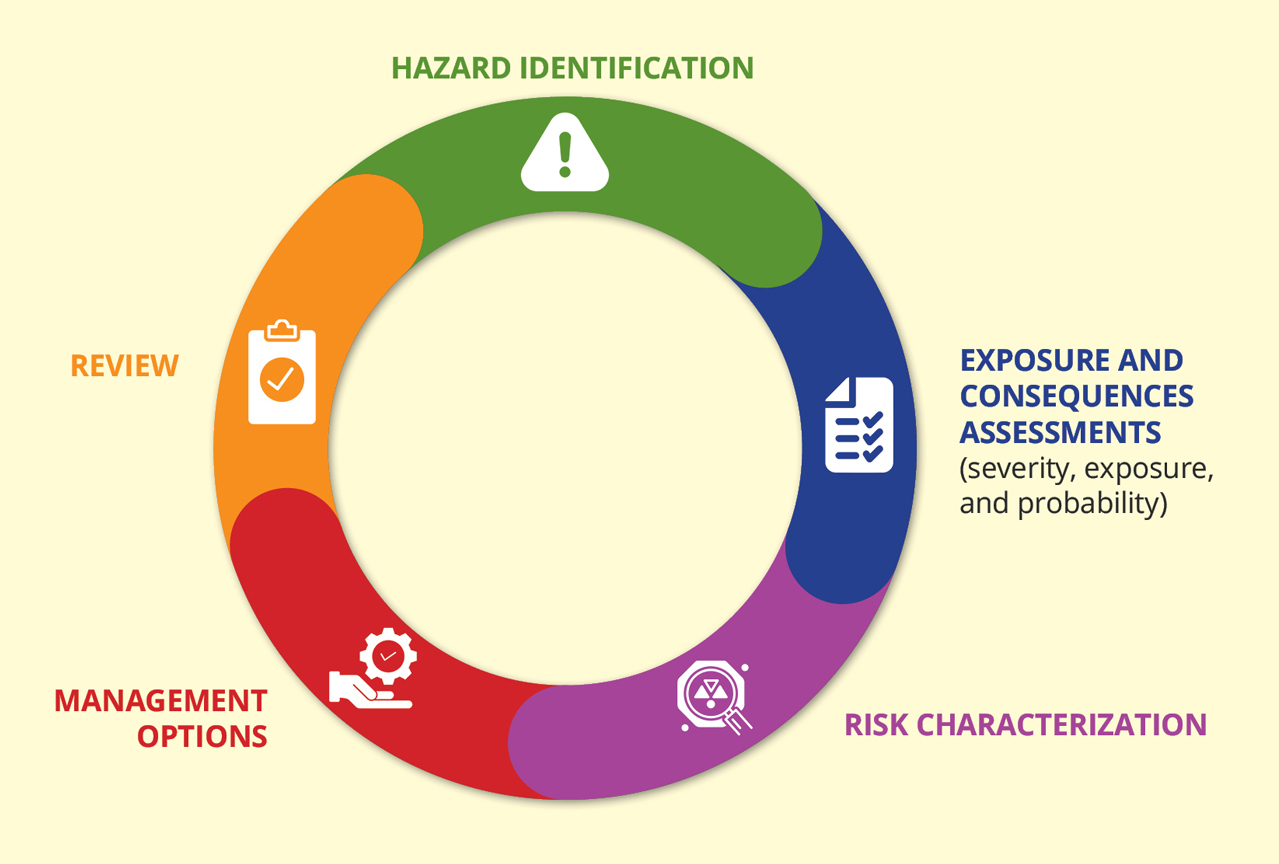
Who oversees gene drive research?
According to the policy brief, gene drive research is regulated at the national level. To ensure they are suitable for new developments and conditions, national authorities can choose to create different or additional requirements aside from the existing ones for other research on genetically modified organisms.
Are we ready to assess the risk and benefits from gene drive technologies?
Many countries have their own draft frameworks for carrying out risk assessments of genetically modified organisms based on international guidelines, such as the Cartagena Protocol on Biosafety. Countries that are in the process of creating their national regulatory frameworks or reviewing them to check their adequacy for evaluating new technologies, international guidance is also helpful. A list of examples of international guidelines is included in the policy brief.
Policy recommendations for risk assessment for gene drives
The policy brief suggests the following recommendations:
- Risk assessment processes must be science-based and consistent with the principle of case-by-case assessment.
- Risk assessment should be inclusive and allow a broad range of stakeholders to voice their concerns and contribute to the process.
- National authorities should turn to existing international risk assessment guidelines for LMOs to create and review their national frameworks.
- Many countries already have strong regulatory frameworks but investments in building biosafety expertise are needed to increase other countries’ ability to take part in and benefit from innovative research.
| Newer Post | Archive | Older Post |
Science Speaks is ISAAA Inc.'s official blog. Weekly blog articles, authored by ISAAA writers, partners, and invited contributors, aim to help share, disseminate, and promote scientific knowledge and its vital role in achieving global agricultural sustainability and development. Your support to Science Speaks will help us achieve this goal. You can help us by donating as little as $10.

