Pocket K No. 42: Stacked Traits in Biotech Crops
| |
The biotech method of gene stacking has brought us many notable products such as “Golden Rice”, “Blue Rose”, and SmartStax™. What is gene stacking and why is there a ‘stack boom’ in the biotech crop market? What does the future hold for biotech stacks?
Knowing the Lingo
Gene stacking refers to the process of combining two or more genes of interest into a single plant. Gene pyramiding and multigene transfer are other monikers in the scientific literature referring to the same process. The combined traits resulting from this process are called stacked traits. A biotech crop variety that bears stacked traits is called a biotech stack or simply stack. An example of a stack is a plant transformed with two or more genes that code for Bacillus thuringiensis (Bt) proteins having different modes of action. It is a hybrid plant expressing both insect resistance and herbicide tolerance genes derived from two parent plants.
Why Gene Stacking
Compared to mono-trait crop varieties, stacks offer broader agronomic enhancements that allow farmers to meet their needs under complex farming conditions. Biotech stacks are engineered to have better chances of overcoming the myriad of problems in the field such as insect pests, diseases, weeds, and environmental stresses so that farmers can increase their productivity.
Gene stacking enhances and simplifies pest management for biotech crops as demonstrated by multiple insect resistance based on Bt gene technology. Experience has shown that the resistance conferred by a single Bt gene has the potential to break down as the target insect pest mutates and adapts to defeat the Bt trait.
To prevent or delay the emergence of resistance to the Bt gene, many regulatory agencies require a refuge or an area planted to a non-Bt variety alongside the Bt crop. Typically a refuge is about 20 percent of the total crop area for a mono-Bt trait variety.
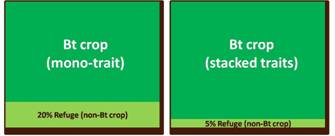
While the refuge strategy lessens the chance for the insect pest to overcome the Bt trait, farmers cannot realize the full production benefit of the Bt crop. The next generation of Bt crops with multiple modes of action for insect control were then developed by stacking several classes of Bt genes. This gene stacking approach has reduced the potential of resistance breakdown as it is more difficult for the pest to overcome multiple insecticidal proteins. This greater durability of Bt stacks allow a lower refuge area requirement that somehow limits yield.1
The Bt gene stacking principle is also used in weed management. Weed resistance to commercial herbicides has been documented for different herbicidal modes of action.2 To catch up in countering weed resistance, biotech seed developers have stacked up genes to broaden the herbicidal mode of actions. For example, this is done by combining the glyphosate resistance gene epsps with the pat gene conferring resistance to herbicide glufosinate and/or with the dmo gene conferring resistance to herbicide dicamba.
Gene stacking is especially useful in metabolic engineering of plants since most metabolic processes and biochemical pathways involve numerous genes interacting with each other.3 For example, the entire pathway for provitamin A (beta carotene) biosynthesis was engineered in the rice endosperm by stacking three carotenoid genes into rice.4 The biotech rose with modified flower color was produced by stacking two genes in the anthocyanin biosynthetic pathway that altered the flower pigmentation process, giving the biotech rose flowers novel shades of blue.5
Gene Stacking Process
Table 1 summarizes the common methods of gene stacking and provides examples of commercialized stacks produced by each method. The easiest and quickest way to stack up genes into a plant is to make crosses between parental plants that have different biotech traits, an approach known as hybrid stacking. Most of the commercially available biotech stacks, like triple stack, and quadruple stack, are products of serial hybrid stacking which is widely adapted and accepted.6 Another method of gene stacking is known as molecular stacking which involves the introduction of gene constructs simultaneously or sequentially into the target plant by standard delivery systems such as Agrobacterium-mediated and biolistic methods.7,8 In some stacks, molecular stacking has been done with conventional breeding approaches to put together the desirable traits.
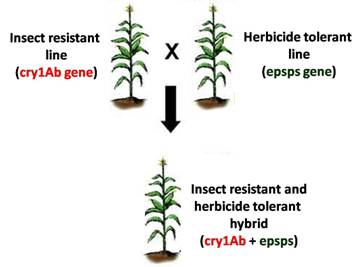
Table 1. Common gene stacking methods used in the production of biotech stacks.
Gene stacking method |
Description |
Examples of commercial stacks* |
Hybrid stacking |
A plant harboring one or more transgenes is cross-hybridized with another plant containing other transgenes. Development of a multi-stack hybrid occurs via iterative hybridization. |
Maize: Agrisure™ Viptera™ 3220 (Bt11 x MIR162 x TC1507 x GA21) Cotton: Roundup Ready™ Flex Bollgard™ II (MON88913 x MON15985) |
Co-transformation |
A plant is transformed with two or more independent transgenes. The transgenes of interest are in separate gene constructs and delivered to the plant simultaneously. |
Maize: NaturGard™ Knockout™ (Bt176), Bt Xtra™ (DBT418), YieldGard™ (MON810, MON809, MON802) |
Linked genes or multigene cassette transformation |
A plant is transformed with a single gene construct that harbors two or more linked transgenes. |
Maize: Herculex™ I (TC1507), Herculex™ RW (59122), Agrisure™ CB/LL (Bt11) Soybean: Vistive™ Gold (MON87705) |
Re-transformation |
A plant harboring a transgene is transformed with other transgenes. |
Cotton: Bollgard™ II (MON15985) |
*The examples of commercial biotech stacks are taken from the online ISAAA GM Approval database6. Links to more detailed information on the derivation of the biotech stacks are provided therein.
Trends in Biotech Stacks
It is estimated that a total of 80.5 million ha were planted to biotech stacks in 2018. This accounts for more than 42 percent of the 191.7 million ha of biotech crops planted worldwide.9 The US Department of Agriculture estimated that 89 percent of cotton acres and 80 percent of corn acres were planted with stacked seeds in 2019.10
The first stack that gained regulatory approval in 1995 was a dual hybrid cotton stack produced by crossing Bollgard™ cotton that expresses the Bt toxin cry1Ab and Roundup Ready™ cotton that produces the epsps enzyme conferring resistance to herbicide glyphosate. Following commercial success of this hybrid stack, developers sought to stack up more biotech traits into their crop portfolio to create multi-stack hybrids. The cotton triple stack which combines two Bt genes with the glyphosate resistance gene occupied more than 54 percent of the US cotton area in 2008. The recently released eight-gene maize stack known by its trade name SmartStax™ is the result of crossing four different biotech maize lines to combine two herbicide tolerance genes with six Bt genes. The resulting stack features dual modes of control for weeds, lepidopteran insects and coleopteran insects and allowed for the refuge requirement to be reduced from 20 percent to 5 percent in the US Corn Belt.11
The increasing number of biotech traits in recent stacks has set the trend for the next generation of biotech crops. Future stacks are likely to involve not only multiple pest resistances but the combination of these traits with engineered metabolic pathways and simultaneous introductions of multiple pathways through metabolic engineering (for example, pathways for beta carotene, ascorbate, folate and vitamin E synthesis).3,7,8
Regulatory Approaches
Regulatory principles and procedures for approval and release of biotech stacks differ globally. In countries like the USA and Canada, no separate or additional regulatory approval is necessary for commercializing hybrid stacks that are products of crossing a number of already approved biotech lines. This policy is based on the argument that interactions between individual trait components in a stack that have been shown to pose no environmental or health hazard would not result in new or altered hazards. The US Environmental Protection Agency, however, may require separate safety review of a stack upon identification of a specific hazard associated with combined “plant incorporated protectants” or PIPs (eg. Bt insecticidal proteins), since combinations of PIPs may result in higher or altered toxicity.
In Japan and European Union (EU) countries to the contrary, stacks are considered new events, even if individual events have market approval, and must pass through a separate regulatory approval process, including risk assessment of their safety, similar to mono-trait biotech events. Risk assessment is focused on the identification of additional risks that could arise from the combined genes. Possible risks are altered effects of interacting proteins on the target and non-target organisms and increased invasiveness of the crop that may pose environmental risks.
Technological Challenges
For the developer, the choice between a large molecular stack or a complex hybrid stack will be based on the monetary cost and timeline for developing and registering a stack. In countries where a stack must pass a separate regulatory review, the one-shot molecular stacking may be more cost effective than the lengthy hybrid stacking. There are, however, technological
concerns in molecular stacking which include the design of large multi-gene constructs, method of delivery into plant cells and the stability of expression of multiple genes. Molecular biologists are developing new genetic engineering approaches to address these concerns. Among the
promising technologies include site-specific gene recombination systems in conjunction with the use of engineered DNA cutting enzymes and the artificial gene assembly known as minichromosome.7,8
How the multiple transgenes might affect the overall physiology of the plant and how many genes and what combinations of genes can be stacked into a plant are of primary concern to
developers. If multiple transgene insertions will alter protein synthesis and metabolic processes of the plant drastically, these likely will compromise the yield. While this yield drag is not necessarily a biosafety concern, the yield loss will need to be offset by reduced production cost and the extra premium farmers will make from the added value of the biotech trait(s) in order for a stack to be a viable and beneficial biotech product.
| |
References
- Storer, N. P., Thompson, G. D. And Head, G. P. (2012) Application of pyramided traits against Lepidoptera in insect resistance management for Bt crops. GM Crops and Food 3:3, 154-162.
- International Survey of Herbicide Resistant Weeds. (2017). Herbicide-Resistant Weeds by Site of Action. http://www.weedscience.org/Summary/SOASummary.aspx.
- Naqvi, S., Farre, G., Sanahuja, G., Capell, T., Zhu, C. and Christou, P. (2009) When more is better: multigene engineering in plants. Trends in Plant Science 15, 48-56
- Ye X.D., Al Babili, S., Kloti, A., Zhang, J., Lucca, P., Beyer, P. and Potrykus, I. (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science, 287, 303–305.
- Tanaka, Y., Brugliera, F and Chandler, S. (2009) Recent Progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 10, 5350 – 5369
- ISAAA. (2017). ISAAA. GM Approval Database. https://www.isaaa.org/gmapprovaldatabase/event/default.asp.
- Halpin, C. (2005) Gene stacking in transgenic plants – the challenge for 21st century plant biotechnology. Plant Biotechnology Journal 3, 141–155
- Que, Q., Chilton, M., de Fontes, C., He, C., Nuccio, M., Zhu, T., Wu, Y., Chen, J. and Shi, L. (2010). Trait stacking in transgenic plants – challenges and opportunities. GM Crops 1:4, 220-229
- ISAAA. (2018). Global Status of Commercialized Biotech/GM Crops: 2018. ISAAA Brief No. 54. ISAAA: Ithaca, NY.
- USDA-ERS. (2019). Recent Trends in GE Adoption. http://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx.
- USDA-EPS. (2015). Pesticide Fact Sheet. https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/smartstax-factsheet.pdf.
* Updated: March 2020
