Pocket K No. 3: Are Food Derived from GM Crops Safe?
| |
|
Using traditional and modern methods superior plant varieties are produced with improved characteristics that make them grow better or more desirable to eat. GM crops are developed using the tools of modern biotechnology where precise tools are used to introduce only the desirable traits into a plant. In contrast, in traditional plant breeding, genes from two parents are mixed in many different combinations in the hope of getting the desired trait. Both methods have the potential to alter the nutritional value of plants or lead to unintended changes in concentration of natural toxicants or anti nutrients. However, these concerns maybe less frequent in transgenic plants since only a limited number of genes are transferred during genetic modification, unlike when traditional breeding methods are used.
Foods derived from GM crops have undergone more testing than any other food in history. Before entering the marketplace, they are assessed using guidelines issued by several international scientific agencies such as the World Health Organization, the Food and Agriculture Organization, and the Organization for Economic Cooperation and Development. These guidelines include the following:
|
- GM food products should be regulated in the same way as foods produced by other methods. The risks associated with foods derived from biotechnology are of the same nature as those for conventional foods.
- These products will be judged on their individual safety, allergenicity, toxicity, and nutrition rather than the methods or techniques used to produce them.
- Any new ingredient added to food through biotechnology will be subject to pre-market approval in the same way a new food additive, such as a preservative or food color, must be approved before it reaches the marketplace.
How are foods derived from GM crops assessed for food safety?
Before any GM food can enter the market, it has to be exhaustively tested by the developer and independently evaluated for safety by scientists or experts in nutrition, toxicology, allergenicity, and other aspects of food science. These food safety assessments are based on guidelines issued by competent regulatory agencies of each country and include: a description of the food product; detailed information about its proposed use; and molecular, biochemical, toxicological, nutritional, and allergenicity data. Typical questions that must be addressed are:
- Does the GM food have a traditional counterpart that has a history of safe use?
- Has the concentration of any naturally occurring toxins or allergens in the food changed?
- Have the levels of key nutrients changed?
- Do new substances in the GM food have a history of safe use?
- Has the food’s digestibility been affected?
- Has the food been produced using accepted, established procedures?
Even after these and other questions about the GM food are answered, there are still more steps in the approval process before the GM food can be commercialized. In fact, GM foods are the most studied food products ever produced.
What are the issues?
Toxicity
In nature, plants contain low concentration of toxins to protect it from insect pests and diseases. A list of many common plant toxins and anti nutrients is available in the Food and Drug Administration of the USA. It has guidelines that determine the normal and acceptable toxin levels of all crops varieties consumed based on toxicological studies. Natural toxin levels of GM crops are similar to their conventional counterparts.
The protein products of the inserted gene in the commercialized GM plants are evaluated in the toxicological tests. Information on anticipated processing conditions that may result in the removal or denaturation of the proteinaceous material is part of the assessment. GM plant products are subjected to acute toxicity studies based on the premise that the mode of action of many known proteins is through acute mechanisms. High doses of purified transgenic proteins which are expressed in bacteria or plant systems are administered orally. This is sufficient to evaluate the toxic potential of the new proteins.
Summary of Acute Toxicity Evaluation of Proteins Introduced in Commercial GM Crops
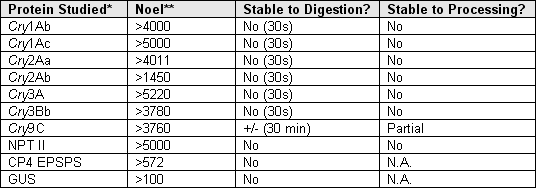
*(1) Cry = crystal protein endotoxins produced by some strains of Bacillus thuringiensis
(2) NPT = neomycin phosphotransferase, a marker enzyme
(3) CP4 EPSPS = 5 enolpyruvylshikimate-3-phosphate synthase gene form Agrobacterium sp. Strain CP4.
(4) GUS = beta glucuronidase reporter gene
** NOEL = No observed adverse effect level.
Toxins of commercialized GM plants are easily digestible in a short time, thus, they are non toxic to humans.
Allergenicity
|
One of the public’s biggest concerns related to GM foods is that an allergen (a protein that causes an allergic reaction) could be accidentally introduced into a food product. There are about 500 amino acid sequences of known protein allergens and 90% of all food allergies are associated with only eight foods or food groups – shellfish, eggs, fish, milk, peanuts, soybeans, tree nuts, and wheat. These, and many other food allergens are well characterized and so it is extremely unlikely that they would ever be introduced into a GM food. A variety of tests and questions must be considered to determine whether the food poses any increased risk of allergenicity
Allergens have shared properties, they are stable during digestion and food processing, and are abundant in foods. Proteins introduced into commercially available GM foods do not have any of these properties. They are from sources with no history of allergenicity or toxicity; do not resemble known toxins or allergens biochemically and structurally; and their functions are well understood. They are also present at very low levels in the GM food, are rapidly degraded in the stomach and have been confirmed as safe in animal feeding studies. The novel proteins in these GM crops have a history of safe use with no allergenic concerns.
The material (DNA) that encodes the genetic information is present in all foods, and its ingestion is not associated with any ill effects. In fact, we take in DNA every time we eat as it is present in all plant and animal material even when it is cooked or raw.
Antibiotic Resistance
|
Some GM crops contain genes such as antibiotic resistance genes to identify cells into which the desired gene has been successfully introduced. Concerns have been raised that these marker genes could move from GM crops to microorganisms that normally reside in a person’s gut and lead to an increase in antibiotic resistance. There have been numerous scientific reviews and experimental studies of this issue and they have come to the following conclusions:
- The likelihood of antibiotic resistance genes moving from GM crops to any other organisms is extremely remote or virtually zero: less than 10-14 to 10-27; and
- Even in the unlikely event that an antibiotic resistance gene is transferred to another organism, the impact of this transfer would be negligible, as the markers used in GM crops have limited clinical or veterinary use.
Nevertheless, in response to public concerns, scientists have been advised to avoid using antibiotic resistance genes in GM plants. Alternative marker strategies are being used in developing the next generation of GM plants (See PK 36).
Substantial Equivalence (SE) in Safety Assessment of GM Foods
Absolute safety is unattainable for any food as people react differently to natural ingredients of food. Substantial equivalence (SE) is an alternative approach used for the safety assessment of genetically modified foods where traditional toxicological testing and risk assessment to whole foods could not be applied. It is based on the idea that existing products used as foods or food sources can serve as basis for comparison. The safety assessment is therefore based on a comparison of the modified food to its traditional (non GM) counterpart in terms of molecular, compositional, toxicological and nutritional data. SE has been used in the safety assessment of GM crops available today.
Mon 810 for example has been compared rigorously as to the levels of major nutritional components (protein, fat, ash, carbohydrates, calories and moisture) with the non transgenic counterpart Mon 818. Results showed that the amino acid composition, fatty acids, inorganic composition (calcium and phosphorous), carbohydrate components (starch, sugars and phytic acid, crude fiber), and tocopherol content of Mon 810 are within the range of Mon 818.
Conclusion
Foods derived from GM plants are safe. Major issues and safety concerns on the biosafety of foods derived from GM plants have been addressed. Protein products of the inserted genes in the commercially available GM plants have passed the rigorous tests and showed that they are non toxic, non-allergenic, and the nutritional content is comparable to their non GM counterpart. GM plants that are being developed also undergo similar testing before they are released commercially.
International agencies such as the Food and Agriculture Organization, World Health Organization, the European Commission, the French Academy of Medicine, the American Medical Association, and the American Society of Toxicology have reviewed these health issues and have come to an agreement that GM foods are safe for human health.
| |
*Updated October 2009
Next Pocket K: GM Crops and the Environment
-
Foldable Version (PDF)
- Bahasa Indonesia
- Chinese
- English
- Filipino
- French (France)
- Hindi
- Portuguese
- Spanish
- Swahili
- Thai
- Urdu
-
Document Version (PDF)
- Chinese
- English
- Vietnamese




