Pocket K No. 22: Plant Disease Diagnostics
| |
Important agricultural crops are threatened by a wide variety of plant diseases and pests. These can damage crops, lower fruit and vegetable quality and wipe out entire harvests. About 42% of the world’s total agricultural crop is destroyed yearly by diseases and pests. Farmers often must contend with more than one pest or disease and new pesticide-resistant pathogenic strains attacking the same crop.
However, crop losses can be minimized, and specific treatments can be tailored to combat specific pathogens if plant diseases are correctly diagnosed and identified early. These need-based treatments also translate to economic and environmental gains.
|
The traditional method of identifying plant pathogens is through visual examination. This is often possible only after major damage has already been done to the crop, so treatments will be of limited or no use. To save plants from irreparable damage by pathogens, farmers have to be able to identify an infection even before it becomes visible.
Is this possible? What happens when pathogens attack a plant? An attack by disease-causing organisms generates a complex immune response in a plant, resulting in the production of disease-specific proteins involved in plant defense and in limiting the spread of infection. Pathogens also produce proteins and toxins to facilitate their infection, before disease symptoms appear. These molecules play vital role in the development of plant diagnostic kits.
Advances in molecular biology, plant pathology, and biotechnology have made the development of such kits possible. These kits are designed to detect plant diseases early, either by identifying the presence of the pathogen in the plant (by testing for the presence of pathogen DNA) or the molecules (proteins) produced by either the pathogen or the plant during infection. These techniques require minimal processing time and are more accurate in identifying pathogens. And while some require laboratory equipment and training, other procedures can be performed on site by a person with no special training.
So far, diagnostic kits have been designed to detect diseases in crops such as rice, potatoes, papaya, tomatoes, and banana. Similar kits are also increasingly important for identifying genetically modified organisms (GMOs) in shipments of conventional crops.
DNA-Based Diagnostic Kits
DNA diagnostic kits are based on the ability of single stranded nucleic acids to bind to other single stranded nucleic acids that are complementary in sequence (referred to as homologous).
The tool used in DNA diagnostic kits is the Polymerase Chain Reaction (PCR). There are 3 steps involved in PCR. The DNA is first unwound, and its strands separated by high temperatures. As the temperature is lowered, short, single-stranded DNA sequences called primers are free to bind to the DNA strands at regions of homology, allowing the (Taq) polymerase enzyme to make a new copy of the molecule. This cycle of denaturation-annealing-elongation is repeated 30-40 times, yielding millions of identical copies of the segment.
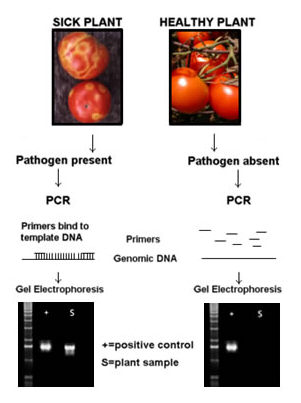
The primers in PCR diagnostic kits are very specific for the genes of a pathogen, and amplification will occur only in diseased plants. (Figure 2)
Figure 1: PCR-based Diagnostic Methods |
|
Source: Alberts, et. al., 1994. |
The primers in PCR diagnostic kits are very specific for the genes of a pathogen, and DNA amplification will occur only in diseased plants. (Figure 1)
Several PCR-based methods have successfully been adapted for plant pathogen detection. Real-time PCR (RT PCR) follows the general principle of polymerase chain reaction; its key feature is that the amplified DNA is quantified, using fluorescent dyes, as it accumulates in the reaction mixture after each cycle. It offers several advantages over normal PCR, including: reduced risk of sample contamination, provision of data in real time and simultaneous testing for multiple pathogens. Real-time PCR protocols are among the most rapid species-specific detection techniques currently available.
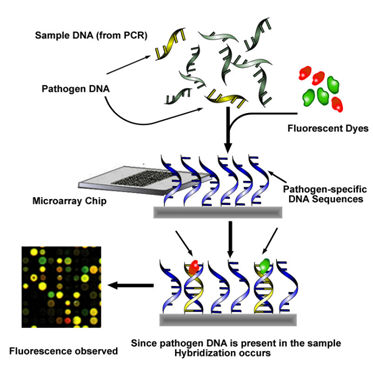
DNA microarrays are also of great use for simultaneous pathogen detection. This is important, as plants are often infected with several pathogens, some of which may act together to cause a disease complex. Microarrays consist of pathogen-specific DNA sequences immobilized onto a solid surface. Sample DNA is amplified by PCR, labeled with fluorescent dyes, and then hybridized to the array (Figure 2).
Figure 2: DNA Microarray |
|
Source: Alberts, et. al., 1994. |
PCR-based diagnostics is very sensitive compared to other techniques; detection of a small amount of DNA is possible. PCR can also help farmers detect the presence of pathogens that have long latent periods between infection and symptom development. Moreover, it can quantify pathogen biomass in host tissue and environmental samples, and at the same time detect fungicide resistance. PCR-based detection, however, is expensive compared to protein-based diagnostic methods, and also requires costly equipments.
So far, PCR kits have been developed to detect black Sigatoka disease in bananas, Phytophthora infestations in potatoes, and Fusarium infection in cotton.
Protein-Based Diagnostic Kits
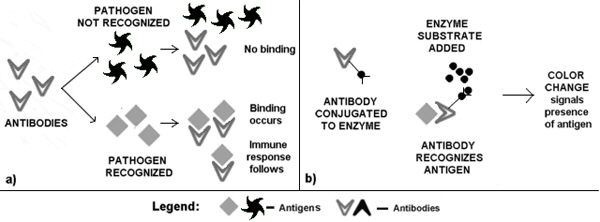
The first step in a defense response reaction is the recognition of an invader by a host’s immune system. This recognition is due to the ability of specific host proteins, called antibodies, to recognize and bind proteins that are unique to a pathogen (antigens) and to trigger an immune reaction (Figure 3a).
Figure 3: Antibody-Antigen Interaction |
|
Alberts, et. al. 1994. |
Protein-based diagnostic kits for plant diseases contain an antibody (the primary antibody) that can either recognize a protein from either the pathogen or the diseased plant. Because the antibody-antigen complex cannot be seen by the naked eye, diagnostic kits also contain a secondary antibody, which is joined to an enzyme. This enzyme will catalyze a chemical reaction that will result in a color change only when the primary antibody is bound to the antigen. Therefore, if a color change occurs in the kit’s reaction mixture, then the plant pathogen is present, (Figure 3b).
The enzyme-linked immunosorbent assay (ELISA) method makes use of this detection system, and forms the basis of some protein-based diagnostic kits. ELISA kits are very easy to use because test takes only a few minutes to perform, and does not require sophisticated laboratory equipment or training.
There are already numerous ELISA test kits available on the market. Some of them detect diseases of root crops (e.g. cassava, beet, potato), ornamentals (e.g. lilies, orchids), fruits (e.g. banana, apple, grapes), grains (e.g. wheat, rice), and vegetables. ELISA techniques can detect ratoon stunting disease of sugarcane, tomato mosaic virus, papaya ringspot virus, banana bract mosaic virus, banana bunchy top virus, watermelon mosaic virus, and rice tungro virus.
One of the first ELISA kits developed to diagnose plant disease was by the International Potato Center (CIP). It can detect the presence of all races, biovars, and serotypes of Ralstonia solanacearum, the pathogen that causes bacterial wilt or brown rot in potato. They also developed a kit that samples for the presence of any of the following sweet potato viruses: SPFMV (sweet potato feathery mottle virus), SPCSV (sweet potato chlorotic stunt crinivirus), SPMSV (Sweet potato mild speckling virus), SPMMV (Sweet potato mild mottle virus), SwPLV (Sweet potato latent virus), SPCFV (Sweet potatochlorotic fleck virus), SPCaLV (Sweet potato caulimovirus), and C-6 (new flexuous rod virus).
Conclusion
With even more advances in molecular biology and immunology, scientists and farmers alike will be able to improve plant disease diagnosis. Efforts are already underway to produce better diagnostic kits to detect pathogens in crops important to developing countries. For instance, the Department of Biotechnology of India’s Ministry of Science and Technology is developing diagnostic kits to detect viruses in fruits, ornamentals, spices, and plantation crops. The Genetic Engineering Services Unit of Egypt’s Agricultural Genetic Engineering Research Institute has developed diagnostic kits and testing services to detect viruses in crop plants.
Diagnostic kits are an investment: they may be expensive, but the costs can be offset by gains, such as reduced crop losses and more environment-friendly crop-management practices. Their development should be made a priority by both the public and private sectors in developing countries.
Glossary
Antibody: Protein produced by immune systems in response to pathogen attack.
Antigen: A substance foreign to a living body that stimulates the production of antibodies. Antigens include proteins, bacteria, and viruses.
ELISA: Enzyme Linked Immunosorbent Assay, a test designed to detect the presence of antigens or antibodies.
PCR: Polymerase Chain Reaction, a technique patterned after DNA replication, where millions of copies of a DNA fragment are produced, making the DNA fragment easier to isolate, clone, and sequence.
Primers: Short, single-stranded DNA fragments designed to be complementary to a region of the genome. Primers are used as the starting point for PCR.
| |
References:
Alberts, et. al. The Molecular Biology of the Cell. 4th ed. 1994.
http://www.cipotato.org/market/ARs/Ar98/InBrief.htm
http://www.agriculture.gov.bb/files/sweet%20potato%20paper.pdf
*October 2008