Enhanced CRISPR-Cas9 Enables Stable Insertion of Large Genes
May 15, 2024| |
Scientists at the Leibniz Institute of Plant Biochemistry (IPB) have successfully inserted large gene segments into the DNA of higher plants very efficiently in a stable and precise manner. The research team optimized CRISPR-Cas9, and the improved method offers great opportunities for the targeted modification of genes in higher plants, both for breeding and research.
CRISPR-Cas has enormous potential for the targeted modification of individual genes. However, this does not apply to all kinds of genetic modifications. While CRISPR is ideal for knocking out genes, they do not work well for precisely inserting genes or replacing gene segments. Targeted insertion of genes into the DNA of higher plants has only been successful in rare individual cases.
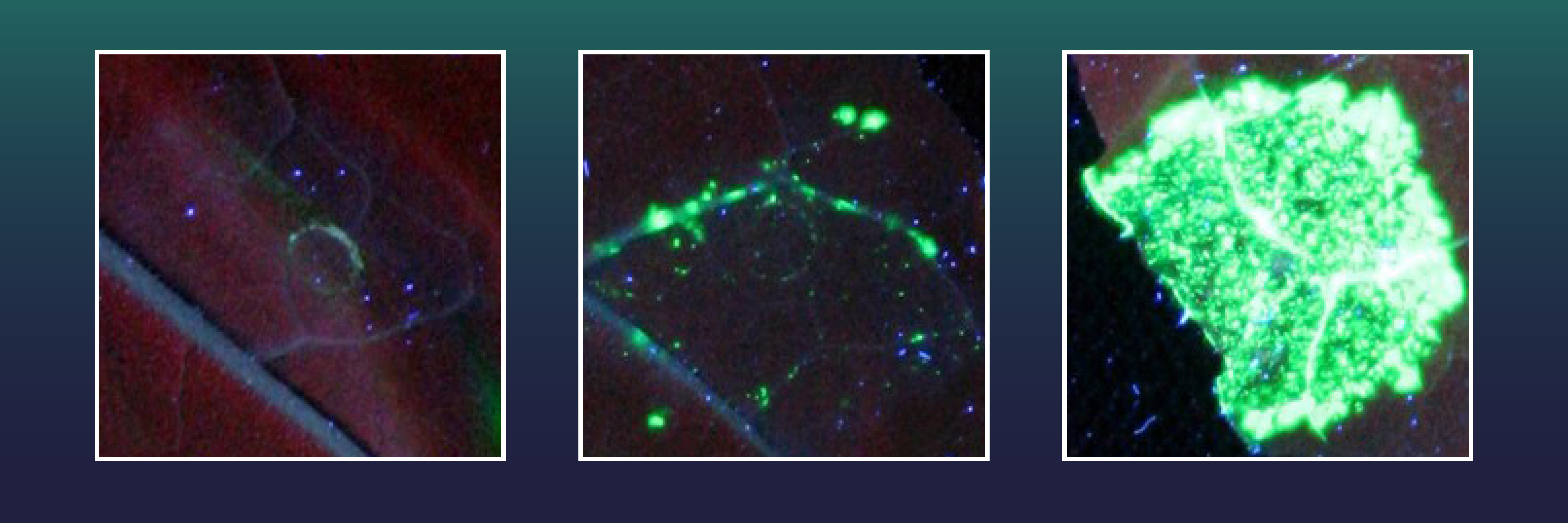
To increase the success rate of gene insertion, the Halle scientists equipped CRISPR-Cas with an additional enzyme, an exonuclease to alter the DNA cleavage sites created by the genetic scissors. The cell's internal repair enzymes can no longer recognize and mend the DNA damage. The DNA segment to be inserted by CRISPR-Cas would therefore have enough time to integrate itself at the correct position through another, very precise, cellular repair mechanism. The Halle scientists tested various exonucleases of viral, bacterial, plant, and human origin for their ability to increase the number of precise gene insertion events. They introduced the genetic scissors with the corresponding exonucleases and a gene X segment into the leaf cells of the tobacco plant Nicotiana benthamiana which had been previously equipped with a gene for a green fluorescent marker.
The green marker can only be produced when the missing gene section of X is precisely reinserted using CRISPR/Cas, thus repairing gene X. Two of the exonucleases tested proved to be particularly effective. Using these, the team from Halle achieved 38 times more perfect gene insertion events than with CRISPR-Cas alone.
For more details, read the news release on the IPB website.
| |
You might also like:
- New CRISPR Tool Allows Precise Inserts of Large DNA Sequences in Targeted Sites
- Study Finds CRISPR-Cas9 Leads to Unexpected Genomic Changes
- CRISPR SWAPnDROP Enables Efficient Large-Scale Interspecies Gene Transfer
Biotech Updates is a weekly newsletter of ISAAA, a not-for-profit organization. It is distributed for free to over 22,000 subscribers worldwide to inform them about the key developments in biosciences, especially in biotechnology. Your support will help us in our mission to feed the world with knowledge. You can help by donating as little as $10.
-
See more articles:
-
Gene Editing Supplement (May 15, 2024)
-
Research and Tools
- China Approves Gene-edited Wheat and GM Maize
- Gene-edited Tomato Exhibits Improved Drought Resistance and Fruit Yield
- Enhanced CRISPR-Cas9 Enables Stable Insertion of Large Genes
- Gene Editing Boosts Soybean Yield and Protein Content
-
Read the latest: - Biotech Updates (May 29, 2024)
- Gene Editing Supplement (May 29, 2024)
- Gene Drive Supplement (February 22, 2023)
-
Subscribe to BU: - Share
- Tweet